Tardigrades could teach us how to handle the rigors of space travel
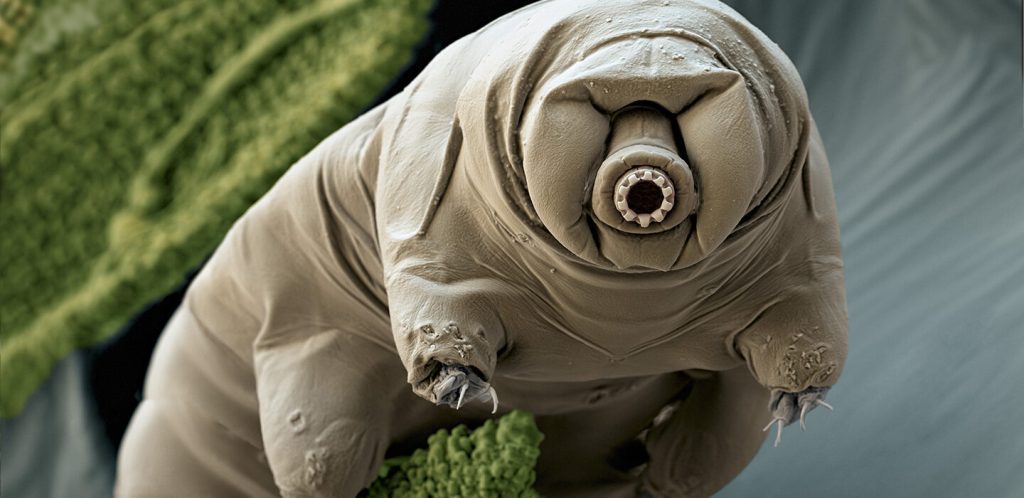
No beast on Earth is tougher than the tiny tardigrade. It can survive being frozen at -272° Celsius, being exposed to the vacuum of outer space and even being blasted with 500 times the dose of X-rays that would kill a human.
In other words, the creature can endure conditions that don’t even exist on Earth. This otherworldly resilience, combined with their endearing looks, has made tardigrades a favorite of animal lovers. But beyond that, researchers are looking to the microscopic animals, about the size of a dust mite, to learn how to prepare humans and crops to handle the rigors of space travel.
The tardigrade’s indestructibility stems from its adaptations to its environment — which may seem surprising, since it lives in seemingly cushy places, like the cool, wet clumps of moss that dot a garden wall. In homage to such habitats, along with a pudgy appearance, some people call tardigrades water bears or, adorably, moss piglets.
But it turns out that a tardigrade’s damp, mossy home can dry out many times each year. Drying is pretty catastrophic for most living things. It damages cells in some of the same ways that freezing, vacuum and radiation do.
For one thing, drying leads to high levels of peroxides and other reactive oxygen species. These toxic molecules chisel a cell’s DNA into short fragments — just as radiation does. Drying also causes cell membranes to wrinkle and crack. And it can lead delicate proteins to unfold, rendering them as useless as crumpled paper airplanes. Tardigrades have evolved special strategies for dealing with these kinds of damage.
As a tardigrade dries out, its cells gush out several strange proteins that are unlike anything found in other animals. In water, the proteins are floppy and shapeless. But as water disappears, the proteins self-assemble into long, crisscrossing fibers that fill the cell’s interior. Like Styrofoam packing peanuts, the fibers support the cell’s membranes and proteins, preventing them from breaking or unfolding.
At least two species of tardigrade also produce another protein found in no other animal on Earth. This protein, dubbed Dsup, short for “damage suppressor,” binds to DNA and may physically shield it from reactive forms of oxygen.
Emulating tardigrades could one day help humans colonize outer space. Food crops, yeast and insects could be engineered to produce tardigrade proteins, allowing these organisms to grow more efficiently on spacecraft where levels of radiation are elevated compared with on Earth.
Scientists have already inserted the gene for the Dsup protein into human cells in the lab. Many of those modified cells survived levels of X-rays or peroxide chemicals that kill ordinary cells (SN: 11/9/19, p. 13). And when inserted into tobacco plants — an experimental model for food crops — the gene for Dsup seemed to protect the plants from exposure to a DNA-damaging chemical called ethyl methanesulfonate. Plants with the extra gene grew more quickly than those without it. Plants with Dsup also incurred less DNA damage when exposed to ultraviolet radiation.
Tardigrades’ “packing peanut” proteins show early signs of being protective for humans. When modified to produce those proteins, human cells became resistant to camptothecin, a cell-killing chemotherapy agent, researchers reported in the March 18 ACS Synthetic Biology. The tardigrade proteins did this by inhibiting apoptosis, a cellular self-destruct program that is often triggered by exposure to harmful chemicals or radiation.
So if humans ever succeed in reaching the stars, they may accomplish this feat, in part, by standing on the shoulders of the tiny eight-legged endurance specialists in your backyard.